Abstract
Introduction: Mutations in the UGT1A1 gene have been implicated in Gilbert's syndrome, which shows mild hyperbilirubinemia, and a more aggressive childhood subtype, Crigler-Najjar syndrome. A common cause of decreased UGT1A1 activity is the insertion of a TA in the TATA box at the promoter region of the UGT1A1 gene which was named as UGT1A1*28 . Individuals with homozygous UGT1A1*28 had higher levels of serum bilirubin compared with those with heterozygous UGT1A1*28 or the wild-type allele.Gilbert's syndrome, also known as constitutional hepatic dysfunction or familial nonhemolytic jaundice, is an inherited disorder of the liver resulting in an overabundance of bilirubin. Most of the patients with Gilbert's syndrome are asymptomatic; however, they sometimes present with episodes of mild intermittent jaundice due to predominantly unconjugated hyperbilirubinemia.Emerging data on the role of genetic variants in the UGT1A1 gene confirm that the UGT1A1*28 allele is associated with severe toxicities in irinotecan-based chemotherapy.Busulfan is an alkylating drug with a narrow therapeutic index which can cause toxicity with higher plasma levels. Recently Mc Donald et al demonstrated in a large cohort of patients that overall mortality and non relapse mortality at 200d after hematopoietic stem cell transplantation were significantly higher in patients with Gilbert's syndrome receiving busulfan-containing conditioning regimens as compared to patients without Gilbert's syndrome without analysis of genomic variants affecting the function of UGT1A1.In a retrospective study we analyzed the role of UGT1A1*28 polymorphism on the transplant related mortality in a cohort of patients submitted to allogeneic stem cell transplantation conditioned with a busulfan-based regimen.
Patients: We evaluated 74 patients (M/F 46/28), median age 48ys (range 21-66), underlying diseases were AML (50 pts), ALL (5 pts), MDS (10 pts), NHL (3 pts), CLL (1 pt), MPN (1 pt) and MM (2 pts). Conditioning regimen used were: BuCy (23 pts), BuFlu (32 pts), TBF (17 pts) and BuMel (2 pts).
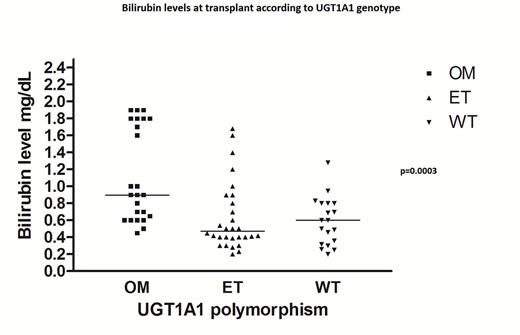
Results: We found 19 wild type (WT) pts (25%), 31 heterozygous (41%) and 24 homozygous pts (32%). Bilirubin levels were significantly higher in homozygous patients as compared to heterozygous and normal both at baseline and during the early follow-up post-transplant (p<0.001 and p=0.01 respectively) (Fig.1). When we looked at correlation between polymorphism and TRM at 200d and at 3ys after transplant it was 15.7% and 17.0% in WT pts, 6.4% and 26.6% in the heterozygous group and 20.8% and 27.3% in the homozygous group respectively (not statistically different).
Conclusions: We demonstrated that patients homozygous for UGT1A1 polymorphism have higher levels of total bilirubin during transplant as compared to WT and heterozygotes but we did not find any correlation between TRM in patients receiving busulfan as part of the conditioning regimen for allo SCT and UGT1A1 polymorphism. Despite this result we are testing the polymorphism on a larger and homogenous cohort of patients because in our opinion it could be useful to identify patients with this genetic mutation responsible of high bilirubin levels at transplant which could be wrongly considered a criteria for diagnosis of VOD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal